What Does a Low Total Bilirubin Level Mean
Abstract
Recent studies have reported that deep white matter lesions (DWMLs) on magnetic resonance imaging scans are related to the risk of developing impaired cognitive function in future. Bilirubin exhibits a potent antioxidant effect and an inverse relationship has been reported between bilirubin levels and the risk of several atherosclerotic diseases; however, there is limited evidence with regard to the effect of bilirubin levels on cerebrovascular diseases including DWMLs. This cross-sectional study included 1121 apparently healthy Japanese adults. The subjects were divided into three groups according to their bilirubin levels (low, <0.5 mg/dl; intermediate, ≥0.5 mg/dl and <1.0 mg/dl; and high, ≥1.0 mg/dl). The severity of DWMLs was evaluated according to Fazekas scale and their relation to bilirubin levels was examined. The association between bilirubin levels and the presence of severe DWMLs was assessed using multivariate logistic regression analysis. The analysis revealed that the low- and intermediate bilirubin groups indicated 2.36- and 1.33-fold increase in the prevalence of severe DWMLs compared with the high-bilirubin group, respectively (95% confidence interval (CI): 1.12–4.97 (the low-bilirubin group), 95% CI: 0.85–2.07 (the intermediate-bilirubin group). In conclusion, low total bilirubin levels could be associated with a high prevalence of severe DWMLs in apparent healthy subjects.
Introduction
An ischemic change in the deep white matter is often documented even in apparently healthy subjects. The deep white matter lesions (DWMLs) are reported to be related to the risk of developing impaired cognitive function and stroke1,2,3. The number of elderly and super-elderly subjects has increased worldwide, particularly in developed countries. Early documentation and intervention may be required in case of such lesions if the subjects are presented with treatable atherosclerotic risk factors. We have formerly reported the strong relationship between DWMLs and visceral-to-subcutaneous fat ratio, which is determined by abdominal computed tomography4. Although the ratio is useful for the risk stratification of cerebral ischemic change, the cost and the exposure to radiation are challenging. Recent studies have indicated a possible relationship between low bilirubin levels and cardiovascular diseases5,6. Similar findings have been reported in the relationship of bilirubin levels with stroke although it remains controversial whether the relationship is causal7,8. In general, bilirubin is an end product of heme metabolism and can be a toxic waste product in subjects with liver impairment such as liver cirrhosis. However, previous studies revealed that bilirubin exhibited potent antioxidant and anti-inflammatory effects9,10. The measurement of serum bilirubin levels is easy, inexpensive, and less invasive. If the relationship between bilirubin levels and DWMLs is confirmed, an early documentation of subjects at a high risk of dementia may be possible. The present study was conducted to examine the effectiveness of bilirubin as a marker for DWHLs.
Results
The Subjects' Characteristics
The present study included 1121 subjects (age, 62 ± 10 years; male, 67%). The background characteristics in terms of the total bilirubin levels are shown in Table 1. There was no significant difference among the groups in terms of atherosclerotic risk factors.
Full size table
The Laboratory Data
The laboratory data are shown in Table 1. There were no significant differences in liver and biliary function tests among the groups. White blood cell count was higher and high-density lipoprotein (HDL) cholesterol was lower in the low-bilirubin group, compared with intermediate- and high- bilirubin groups. The levels of uric acid, an antioxidant product, were similar among the groups.
The Prevalence of Severe DWMLs and the Related Variables
The overall prevalence of severe DWMLs was 20.3%. Of these, the prevalence of the low-, intermediate-, and high-bilirubin groups was 24.1%, 21.3%, and 15.0%, respectively. Figure 1 shows the relationship between the prevalence of severe DWMLs and the total bilirubin levels, wherein a higher prevalence of severe DWMLs was observed in those with lower total bilirubin levels. Univariate logistic regression analysis revealed that age, hemoglobin A1c (HbA1c), systolic blood pressure (SBP), and past smoker were the factors associated with a higher prevalence of severe DWMLs, whereas alcohol consumption was related to a lower prevalence (Table 2). The prevalence of severe DWMLs increased as bilirubin levels decreased (p for trend = 0.026). The intermediate- and low-bilirubin groups exhibited a 1.53 (95% confidence interval (CI) = 1.02–2.29)- and 1.80 (95% CI = 0.96–3.36)-fold increase in the prevalence of severe DWMLs in comparison to the high-bilirubin group. After adjustment for multi-variables, the intermediate- and low-bilirubin groups exhibited a 1.33 (95% CI = 0.85–2.07)- and 2.36 (95% CI = 1.12–4.97)-fold increase in the prevalence of severe DWMLS, although the statistical significance of the linear trend test was attenuated (p for trend = 0.058).
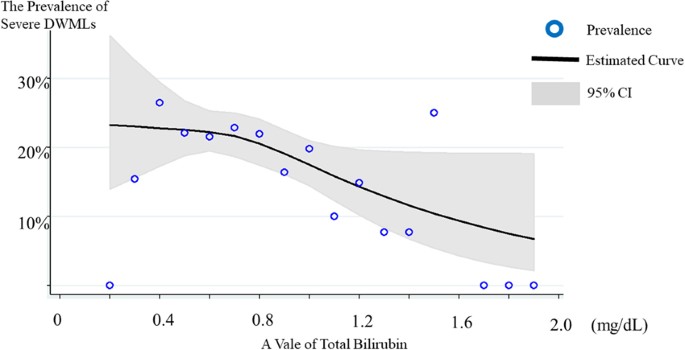
The association between total bilirubin levels and severe DWMLs. The cubic spline curve revealed an inverse association between bilirubin levels and severe DWMLs. DWML: deep white matter lesions.
Full size image
Full size table
The Prevalence of PVH (periventricular hyperintensities) and the Related Variables
Severe PVH was observed in 84 subjects (7.5%) and not related to bilirubin in the uni- and multivariate logistic regression analysis.
Interrater Reliability Regarding Rating of DWMLs and PVH
To evaluate interrater reliability of rating, 150 subjects were randomly selected. Weighted kappa statistics indicated almost perfect agreement (DWMLs: kappa = 0.87 ± 0.06; PVH: kappa = 0.80 ± 0.05, respectively).
Discussion
Previous studies have demonstrated an inverse relationship between serum bilirubin levels and the prevalence of cardiovascular diseases11,12,13,14,15. The present study is the first report that reveals low total bilirubin levels could be associated with a high prevalence of severe DWMLs, which are related to impaired cognitive function, in apparently healthy subjects. Although bilirubin has antioxidant and anti-inflammatory properties and may suppress the development of atherosclerosis in vitro 9,15, these observational studies are not sufficient to determine whether bilirubin would play such roles in the clinical setting as well.
A previous study, which analyzed the association of bilirubin with atherosclerotic risk factors using instrumental variable (IV) method, demonstrated that bilirubin levels were inversely associated with cardiovascular risk factors including body mass index (BMI), HDL cholesterol, and total cholesterol7. The findings suggested that bilirubin might be a confounding factor. Another study which assessed the causal effect of bilirubin levels on stroke risk using the IV method showed no causal relationship8. However, recently, a basic research was conducted to determine whether bilirubin could prevent atherosclerosis16. Vogel ME, et al. elucidated that the administration of bilirubin to low-density lipoprotein receptor-deficient mice impeded plaque formation and significantly reduced the infiltration of monocytes and lymphocytes into aortic root lesions by inhibiting endothelial vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1). Furthermore, they demonstrated that bilirubin blocked VCAM-1- and ICAM-1-mediated migration of monocytes across activated endothelial monolayers16. In the present study, as the inverse relationship was strengthened after adjustment for many possible confounding factors, the protective effect of bilirubin on DWMLs is considered a possibility although its efficacy may differ according to each vessel lesion. Currently, the findings concerning the causal effect of bilirubin are conflicting; thus, further studies are needed.
Serum bilirubin level is measurable in daily clinical practice around the world. We usually evaluate it in case of liver diseases or a suspicion of liver diseases. However, our study suggests that serum bilirubin level is an excellent potential marker for estimating the risk of severe DWMLs, which could be related to the development of stroke and dementia.
As the present study is a cross-sectional study, the causal relationship between bilirubin levels and DWMLs is unknown. However, as mentioned earlier, evaluation of bilirubin may be useful for an early documentation of the risk of dementia. The reason why the relationship between severe PVH and bilirubin was not observed may be due to insufficient number of the subjects with severe PVH.
In conclusion, bilirubin levels <0.5 mg/dl may indicate a higher prevalence of severe DWMLs in apparently healthy subjects.
Methods
Study Design and Population
This cross-sectional study included 1356 Japanese adults aged 40 years or older who were apparently healthy and initiatively underwent a health checkup program focusing on atherosclerosis between 2007 and 2011 at Tokyo Saiseikai Central Hospital. Although the evaluation of cognitive ability was not performed in the health checkup, the presence of cognitive impairment among the participants was unlikely because of their active participation in the health checkup and the capability of completing a self-reported questionnaire. Of these, individuals with abnormal liver enzymes (defined as aspartate transferase > 40 IU/L, alanine transaminase > 40 IU/L, or gamma-glutamyl transpeptidase > 90 IU/L) were excluded because of the possibility of having liver disease. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki in line with the Ethical Guidelines for Epidemiological Research by the Japanese government. The study was approved by the ethics committee of Saiseikai Central Hospital (approval number 345). According to the guidelines, the study satisfied the conditions to waive the requirement for informed consent to individual participants. Therefore, informed consent was waived, and the ethics committee approved the waiver.
Image modalities and conditions
The health checkup was performed focusing on atherosclerosis. Magnetic resonance imaging was performed to evaluate cerebral atherosclerosis using a 1.5-T scanner (SIGNA HDxt 1.5 T; GE Healthcare Japan, Tokyo, Japan). Hyperintensities on T2-weighted and FLAIR images of deep white matter were defined as DWMLs, indicating small-vessel disease. Periventricular hyperintensities on T2-weighted and FLAIR images was defined as PVH. Severity of DWMLs was rated on a scale of 0–3 according to the Fazekas scale: 0 refers to no white matter lesion, 1 refers to the presence of focal lesions, 2 refers to the beginning of confluent lesions, and 3 refers to the presence of diffuse involvement of the entire white matter17,18. Severe DWMLs were defined as lesions with Fazekas grade 3, which is reported to be associated with cognitive impairment19. We evaluated PVH grade according to Fazekas classification: 0 refers to absent, 1 refers to caps or pencil-thin lining, 2 refers to smooth halo, 3 refers to irregular PVH extending into the deep white matter18. PVH Fazekas with grade 3 was defined as severe PVH20. All ratings were performed by physicians under the direction of board certificated radiologist blind to the clinical data.
The diagnosis of risk factors for atherosclerosis
Diabetes mellitus was diagnosed on the basis of the following criteria: self-reporting diabetes or the use of anti-diabetic medications based on the questionnaire, fasting plasma glucose ≥ 126 mg/dl, or HbA1c ≥ 6.5%21. Dyslipidemia was diagnosed if one of the following criteria was fulfilled: low-density lipoprotein cholesterol (calculated as total cholesterol – HDL cholesterol – triglyceride/5) ≥ 140 mg/dl, HDL cholesterol < 40 mg/dl, or triglyceride ≥ 150 mg/dl. Hypertension was defined as a SBP ≥ 140 mmHg or a diastolic blood pressure ≥ 90 mmHg22. Hyperuricemia was defined as a high serum uric acid value ≥ 7.0 mg/dl.
Classification of Total Bilirubin
Total bilirubin was classified by every 0.5 mg/dl as following: low, <0.5 mg/dl; intermediate, ≥0.5 mg/dl and <1.0 mg/dl; and high, ≥1.0 mg/dl. We also analyzed the same population using tertiles of total bilirubin: low, <0.7 mg/dl; intermediate, ≥0.7 mg/dl and <0.9 mg/dl; high, ≥0.9 mg/dl. However, the analysis with tertile categorization might lack clinical relevance since the cutoffs vary according to the distribution of total bilirubin levels. In the present study, we demonstrated the result with more clinically relevant cutoffs. The result obtained by the tertile categorization was demonstrated in eTable 1.
Statistical Analysis
Numerical data are presented as mean ± standard deviation. Categorical variables are expressed as absolute numbers (percentages). Continuous variables were analyzed using the unpaired Student's t-test. The Fisher's exact test and the χ2 test were used to analyze categorical variables. The associations between patients' characteristics and the presence of severe DWMLs were assessed using uni- and multivariate logistic regression analyses and expressed in terms of odds ratio (OR), 95% CI, and P value. Clinically relevant variables (even without statistically significant differences) and variables with a p value < 0.10 in the univariate logistic regression analyses were used in the multivariate logistic regression analysis to determine whether bilirubin is an independent predictor of severe DWMLs. Consequently, in the multivariate logistic regression analyses, OR was adjusted for age, gender, BMI, HbA1c, SBP, smoking (never, past, and current smoker), the frequency of alcohol consumption (never, 1–4 days a week, ≥5 days a week), a natural logarithm of white blood cell counts, hemoglobin, albumin, uric acid, HDL cholesterol, and low-density lipoprotein cholesterol. In the multiple logistic regression, we did not stratify the analysis by gender, because no significant effect modification on the relationship by gender was observed. In this process, models with and without the interaction term of gender and each bilirubin group were constructed and compared using a chi-squared log-likelihood ratio test (χ2 = 0.78, p = 0.68), which found no significant effect modification. The similar uni- and multivariate logistic regression analysis were conducted regarding the prevalence of PVH. Weighted kappa statistics was used to examine interrater reliability between two raters regarding rating of DWMLs and PVH. Statistical significance was set at p < 0.05. All statistical analyses were carried out using Stata software, version 14 (StataCorp, College Station, TX).
References
- 1.
Garde, E., Mortensen, E. L., Krabbe, K., Rostrup, E. & Larsson, H. B. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet 356, 628–634 (2000).
CAS Article PubMed Google Scholar
- 2.
Verdelho, A. et al. White matter changes and diabetes predict cognitive decline in the elderly: the LADIS study. Neurology 75, 160–167 (2010).
CAS Article PubMed Google Scholar
- 3.
Debette, S. & Markus, H. S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 341, c3666 (2010).
Article PubMed PubMed Central Google Scholar
- 4.
Higuchi, S., Kabeya, Y. & Kato, K. Visceral-to-subcutaneous fat ratio is independently related to small and large cerebrovascular lesions even in healthy subjects. Atherosclerosis 259, 41–45 (2017).
CAS Article PubMed Google Scholar
- 5.
Djousse, L. et al. Total serum bilirubin and risk of cardiovascular disease in the Framingham offspring study. Am J Cardiol 87, 1196-1200; A1194, 1197 (2001).
- 6.
Kang, S. J. et al. Elevated serum bilirubin levels are inversely associated with coronary artery atherosclerosis. Atherosclerosis 230, 242–248 (2013).
CAS Article PubMed Google Scholar
- 7.
McArdle, P. F. et al. Association between bilirubin and cardiovascular disease risk factors: using Mendelian randomization to assess causal inference. BMC Cardiovasc Disord 12, 16 (2012).
CAS Article PubMed PubMed Central Google Scholar
- 8.
Lee, S. J. et al. Bilirubin and Stroke Risk Using a Mendelian Randomization Design. Stroke 48, 1154–1160 (2017).
CAS Article PubMed Google Scholar
- 9.
Stocker, R., Yamamoto, Y., McDonagh, A. F., Glazer, A. N. & Ames, B. N. Bilirubin is an antioxidant of possible physiological importance. Science 235, 1043–1046 (1987).
ADS CAS Article PubMed Google Scholar
- 10.
Wu, T. W., Fung, K. P., Wu, J., Yang, C. C. & Weisel, R. D. Antioxidation of human low density lipoprotein by unconjugated and conjugated bilirubins. Biochem Pharmacol 51, 859–862 (1996).
CAS Article PubMed Google Scholar
- 11.
Schwertner, H. A., Jackson, W. G. & Tolan, G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem 40, 18–23 (1994).
CAS PubMed Google Scholar
- 12.
Breimer, L. H., Wannamethee, G., Ebrahim, S. & Shaper, A. G. Serum bilirubin and risk of ischemic heart disease in middle-aged British men. Clin Chem 41, 1504–1508 (1995).
CAS PubMed Google Scholar
- 13.
Djousse, L., Rothman, K. J., Cupples, L. A., Levy, D. & Ellison, R. C. Effect of serum albumin and bilirubin on the risk of myocardial infarction (the Framingham Offspring Study). Am J Cardiol 91, 485–488 (2003).
CAS Article PubMed Google Scholar
- 14.
Hopkins, P. N. et al. Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease. Arterioscler Thromb Vasc Biol 16, 250–255 (1996).
CAS Article PubMed Google Scholar
- 15.
Lin, J. P. et al. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation 114, 1476–1481 (2006).
CAS Article PubMed Google Scholar
- 16.
Vogel, M. E., Idelman, G., Konaniah, E. S. & Zucker, S. D. Bilirubin Prevents Atherosclerotic Lesion Formation in Low-Density Lipoprotein Receptor-Deficient Mice by Inhibiting Endothelial VCAM-1 and ICAM-1 Signaling. J Am Heart Assoc 6 (2017).
- 17.
Wahlund, L. O. et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 32, 1318–1322 (2001).
CAS Article PubMed Google Scholar
- 18.
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I. & Zimmerman, R. A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 149, 351–356 (1987).
CAS Article PubMed Google Scholar
- 19.
Resende, E. P. F. et al. Ischemic cerebrovascular burden evaluated by magnetic resonance imaging in an elderly Brazilian community: The Pieta study. eNeurologicalSci 5, 30–34 (2016).
Article PubMed PubMed Central Google Scholar
- 20.
Yamawaki, M. et al. Association of cerebral white matter lesions with cognitive function and mood in Japanese elderly people: a population-based study. Brain Behav 5, e00315 (2015).
Article PubMed PubMed Central Google Scholar
- 21.
Standards of medical care in diabetes–2014. Diabetes Care 37 Suppl 1, S14–80 (2014).
- 22.
James, P. A. et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311, 507–520 (2014).
ADS CAS Article PubMed Google Scholar
Download references
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 17K18085.
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Higuchi, S., Kabeya, Y., Uchida, J. et al. Low Bilirubin Levels Indicate a High Risk of Cerebral Deep White Matter Lesions in Apparently Healthy Subjects. Sci Rep 8, 6473 (2018). https://doi.org/10.1038/s41598-018-24917-8
Download citation
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/s41598-018-24917-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
What Does a Low Total Bilirubin Level Mean
Source: https://www.nature.com/articles/s41598-018-24917-8
0 Response to "What Does a Low Total Bilirubin Level Mean"
Post a Comment